39 label the phase diagram of pure solvent and a solution
CH150: Chapter 7 – Solutions – Chemistry - Western Oregon … 7.1 Introduction: Recall from Chapter 1 that solutions are defined as homogeneous mixtures that are mixed so thoroughly that neither component can be observed independently of the other. Solutions are all around us. Air, for example, is a solution. If you live near a lake, a river, or an ocean, that body of water is not pure H 2 O but most probably a solution. Question : Label the phase diagram of pure solvent and a solution. - Chegg Expert Answer. 100% (137 ratings) This is …. View the full answer. Transcribed image text: Label the phase diagram of pure solvent and a solution.
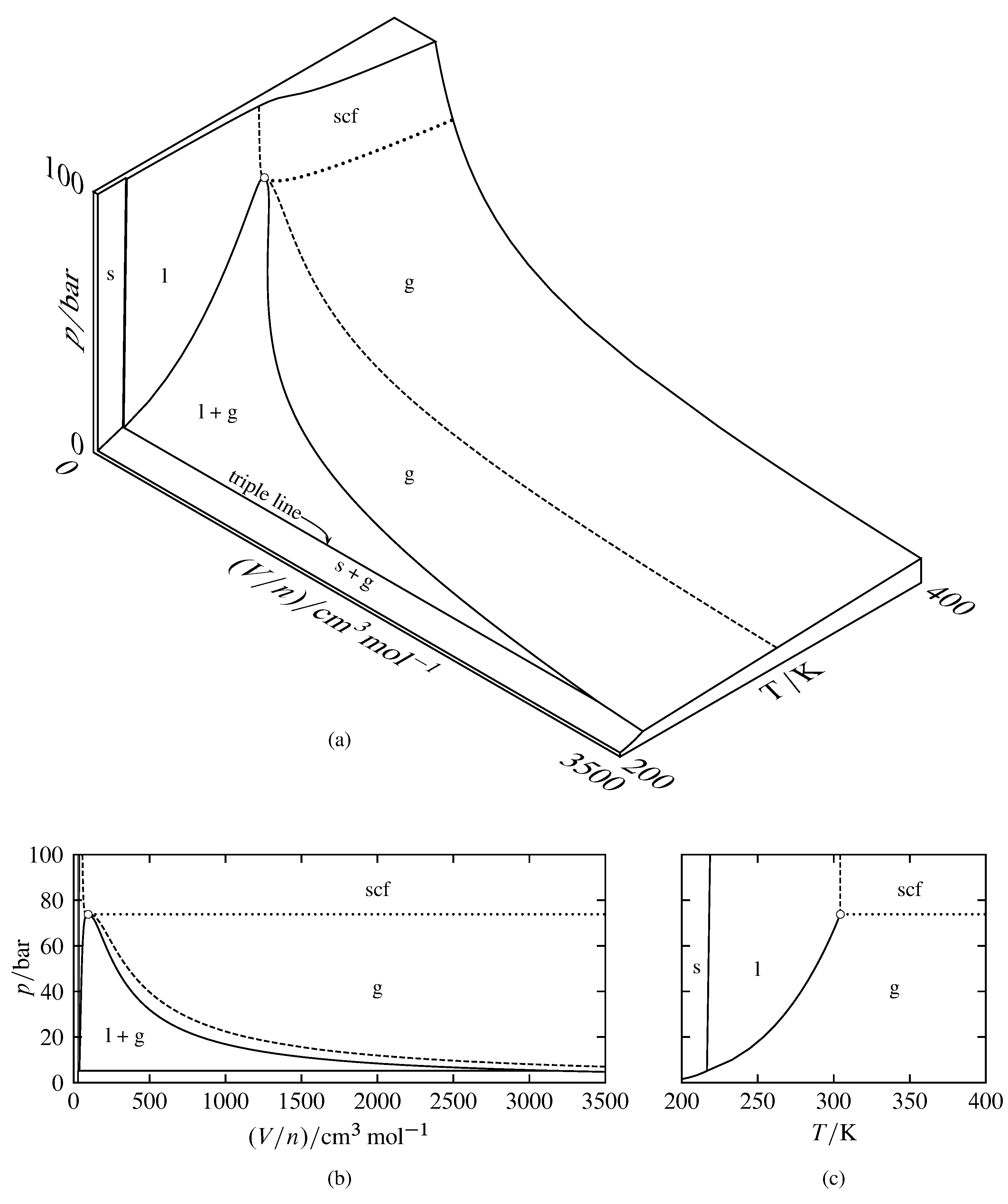
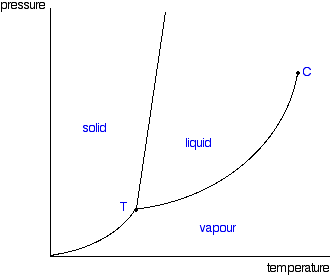
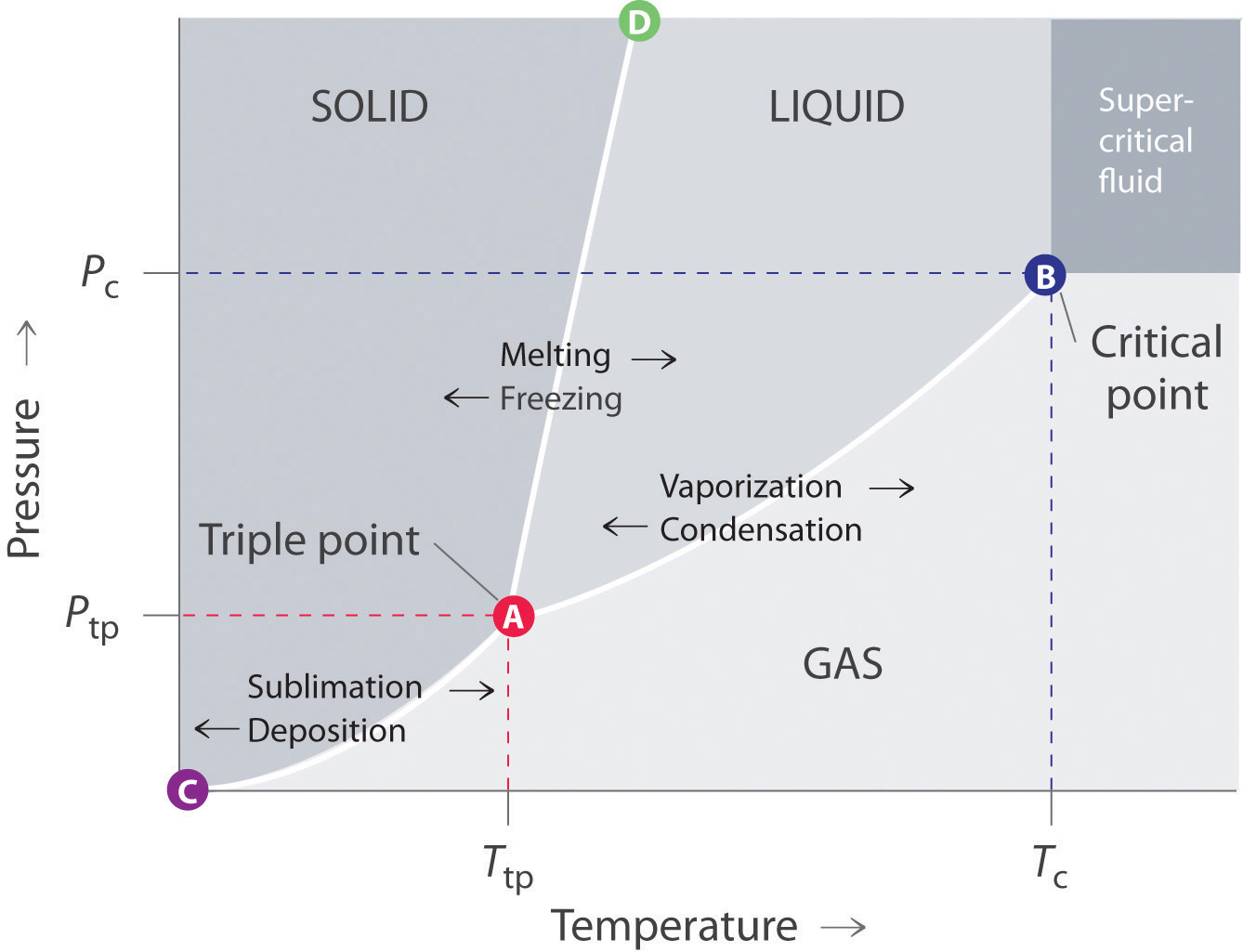
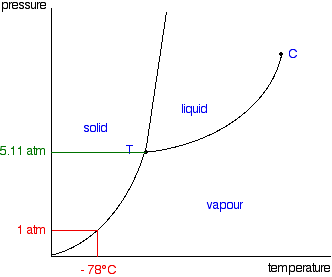
phase diagrams of pure substances - chemguide In the cases we'll be looking at on this page, the phases will simply be the solid, liquid or vapour (gas) states of a pure substance. This is the phase diagram for a typical pure substance. These diagrams (including this one) are nearly always drawn highly distorted in order to see what is going on more easily.
Label the phase diagram of pure solvent and a solution
Phase Diagram | Explanation, Definition, Summary & Facts The phase diagram of a substance can be used to identify the physical and chemical properties of that substance. Here, we will study a general phase diagram by considering different values of one variable while keeping the other variable value constant. In a phase diagram temperature values are drawn on x-axis, whereas pressure values on y-axis. An overview on the use of additives and preparation procedure in phase … In the context of long-term energy storage, solid-to-solid PCMs are recently garnering considerable attention owing to the discovery of materials, mainly plastic crystals, with high enthalpy of transition between the solid phases .They avoid the liquid phase and offer several advantages in comparison to the solid-to-liquid PCMs, such as lack of leakages and of … Rearrangement - Michigan State University In the following diagram, the simplest hypervalent carbocation, methanonium, is drawn on the left in the gray shaded box. This ion is commonly seen in the mass spectrum of methane (gas phase), but decomposes in solution as a consequence of its extreme acidity. To its right are two larger non-classical ions, 2-norbornyl and 7-norbornenyl.
Label the phase diagram of pure solvent and a solution. Phase Diagrams - Lardbucket.org Figure 11.22 A Typical Phase Diagram for a Substance That Exhibits Three Phases—Solid, Liquid, and Gas—and a Supercritical Region Note the Pattern The solid phase is favored at low temperature and high pressure; the gas phase is favored at high temperature and low pressure. General Features of a Phase Diagram Patricia LOSADA-PÉREZ | Professor | Université Libre de Bruxelles ... The critical region in the phase diagram of condensed matter systems such as fluids or fluid mixtures is characterized by the anomalous behaviour of specific thermodynamic properties. solid-liquid phase diagrams: salt solution - chemguide The phase diagram for sodium chloride solution. What the phase diagram looks like. ... because adding a non-volatile solute to a solvent increases its boiling point. ... you could read the page about the phase diagrams of pure substances. Again, it isn't essential to understanding the rest of this page. You just need to be aware that the line ... Answered: 1) Label the LLE diagram provided… | bartleby 1) Label the LLE diagram provided indicating which component is the solvent and which is cariet, and identify the solvent rich (extract) phase boundary and the raffinate phase boundary. 2) Identify Each of the (mark as 'a', 'b', etc) following points and indicate types and number of phases present in each composition. a.
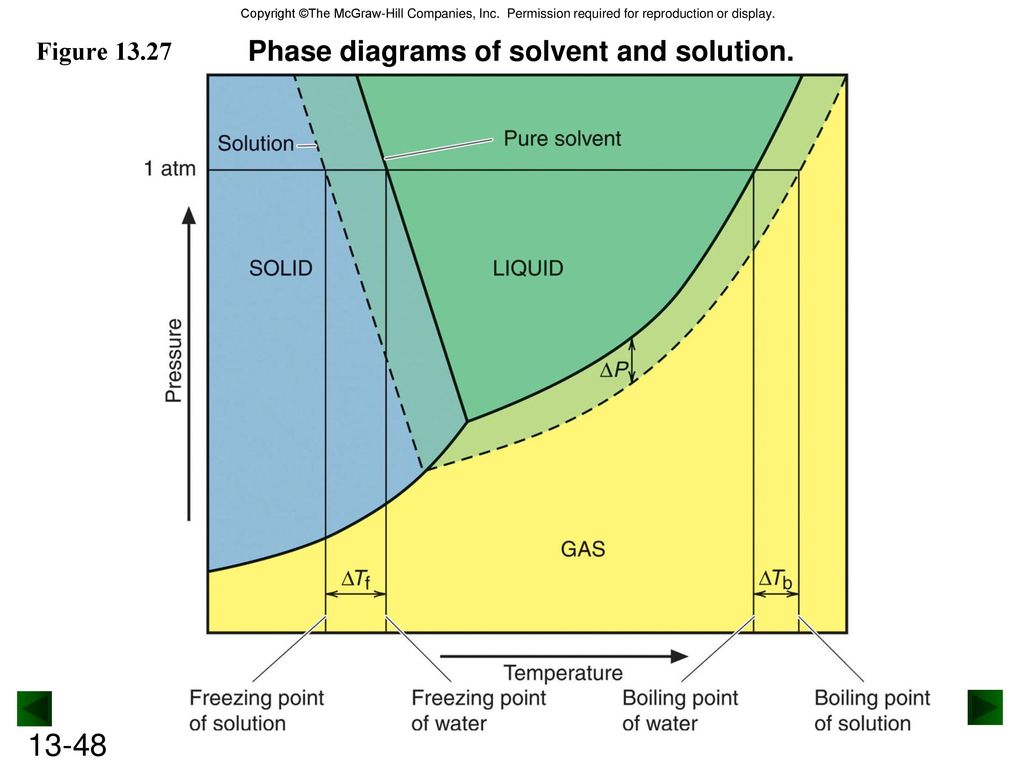
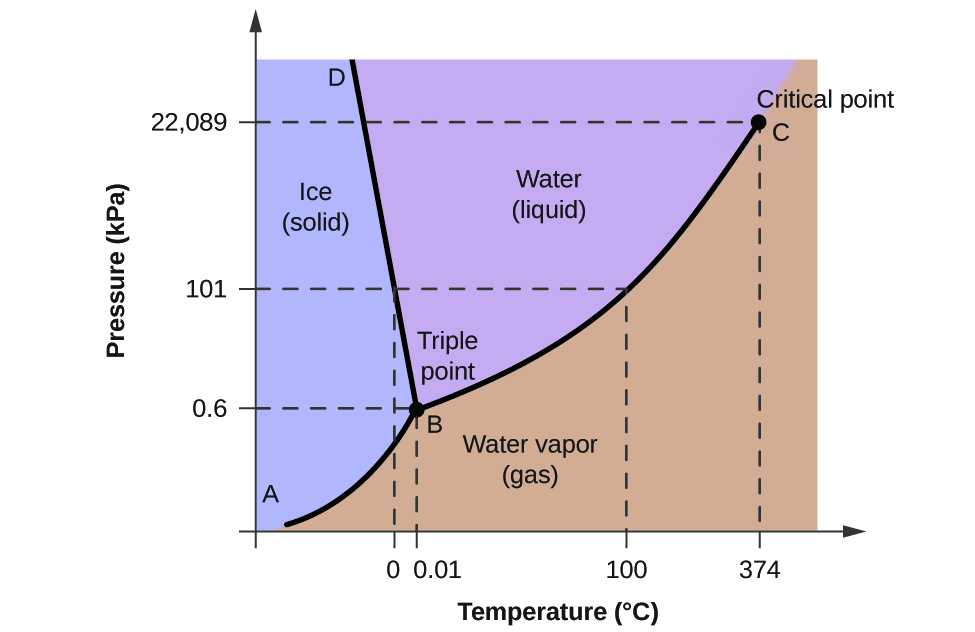
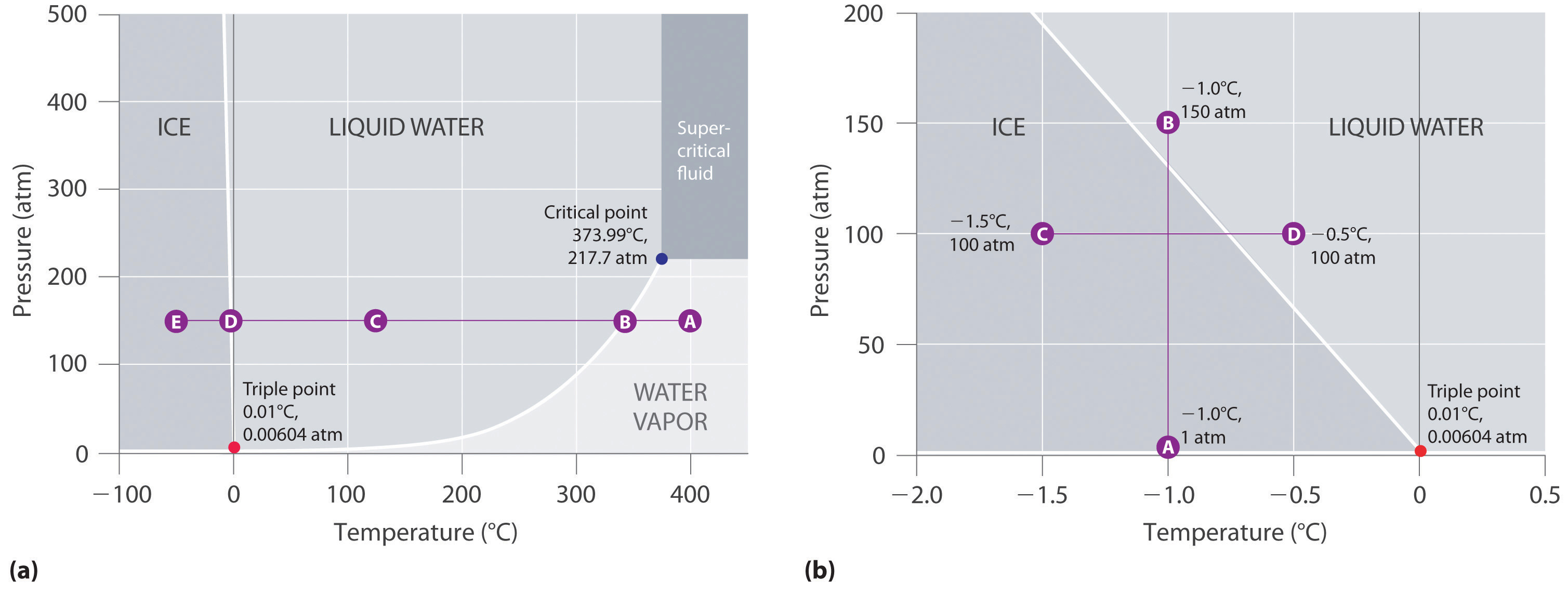
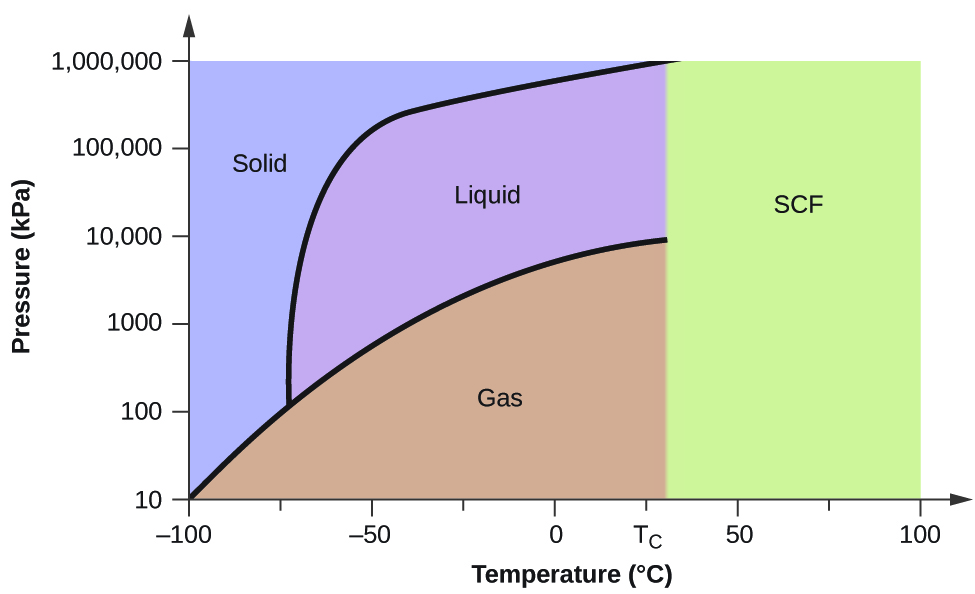
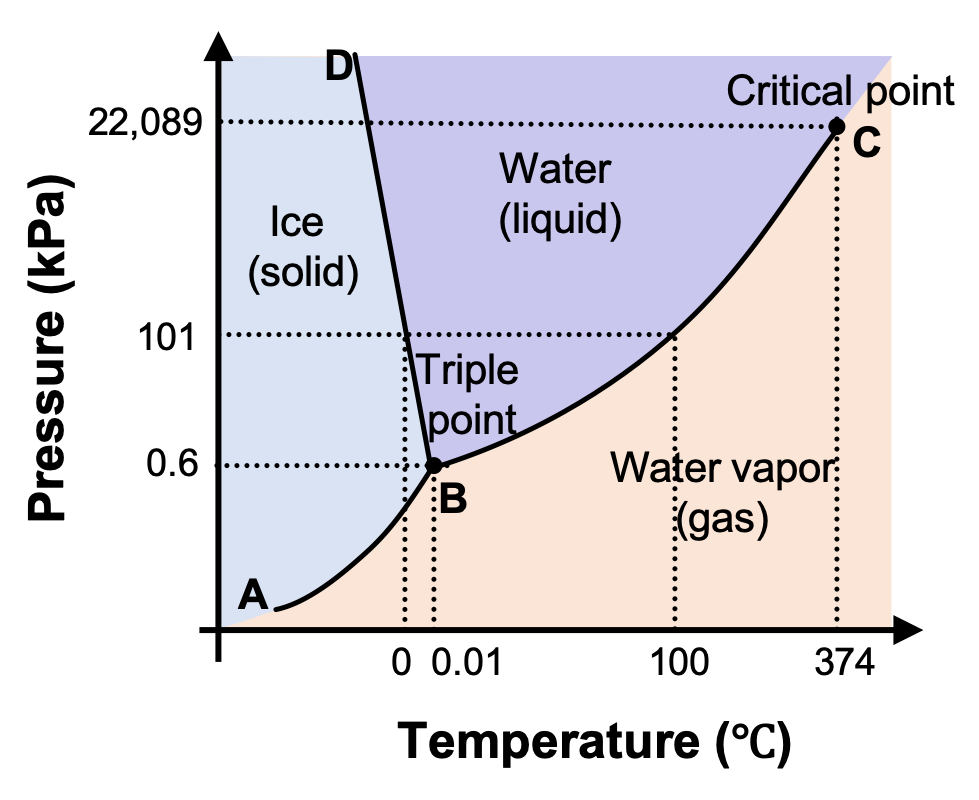
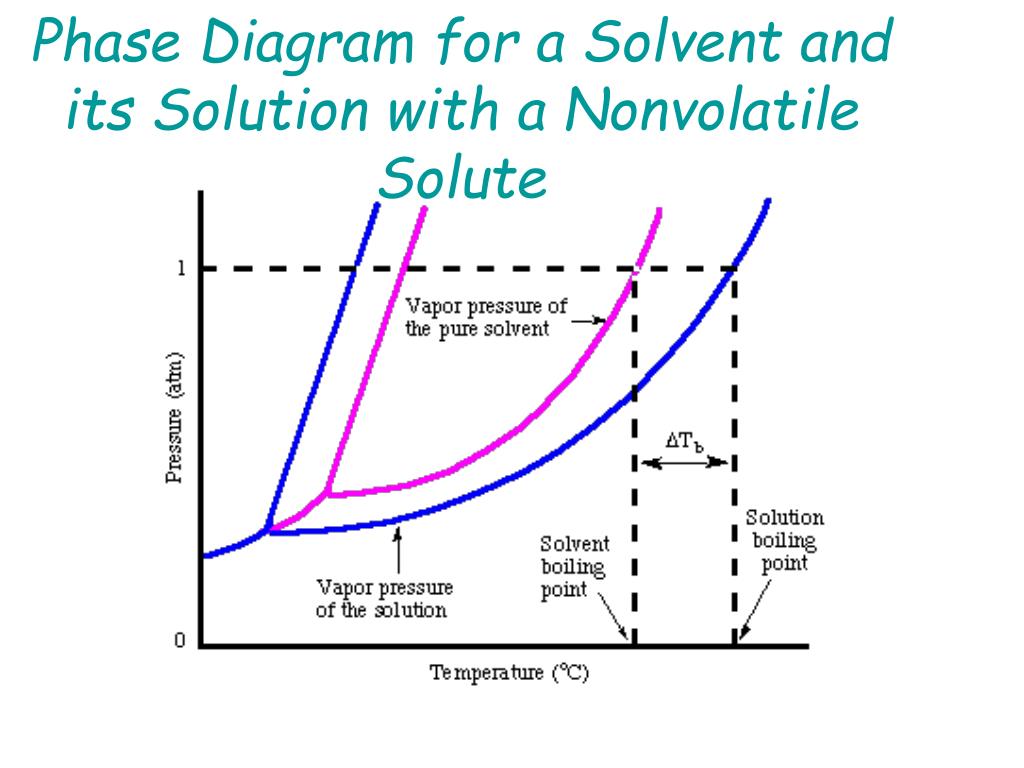
PDF Phase Diagrams When a second compound is introduced to the system forming a homogeneous solution however, the phase diagram drastically changes. For example, the addition of a solute to a pure solvent (making a solution) can disrupt important interactions between solvent molecules, changing the temperature at which the solvent would typically freeze or boil. Phase Diagrams | Chemistry for Majors - Lumen Learning A typical phase diagram for a pure substance is shown in Figure 1. Figure 1. The physical state of a substance and its phase-transition temperatures are represented graphically in a phase diagram. To illustrate the utility of these plots, consider the phase diagram for water shown in Figure 2. Figure 2. Phase Diagrams | Chemistry - Lumen Learning A typical phase diagram for a pure substance is shown in Figure 1. To illustrate the utility of these plots, consider the phase diagram for water shown in Figure 2. Figure 2. The pressure and temperature axes on this phase diagram of water are not drawn to constant scale in order to illustrate several important properties. 10.4 Phase Diagrams - General Chemistry 1 & 2 A typical phase diagram for a pure substance is shown in Figure 1. Figure 1. The physical state of a substance and its phase-transition temperatures are represented graphically in a phase diagram. To illustrate the utility of these plots, consider the phase diagram for water shown in Figure 2. Figure 2.
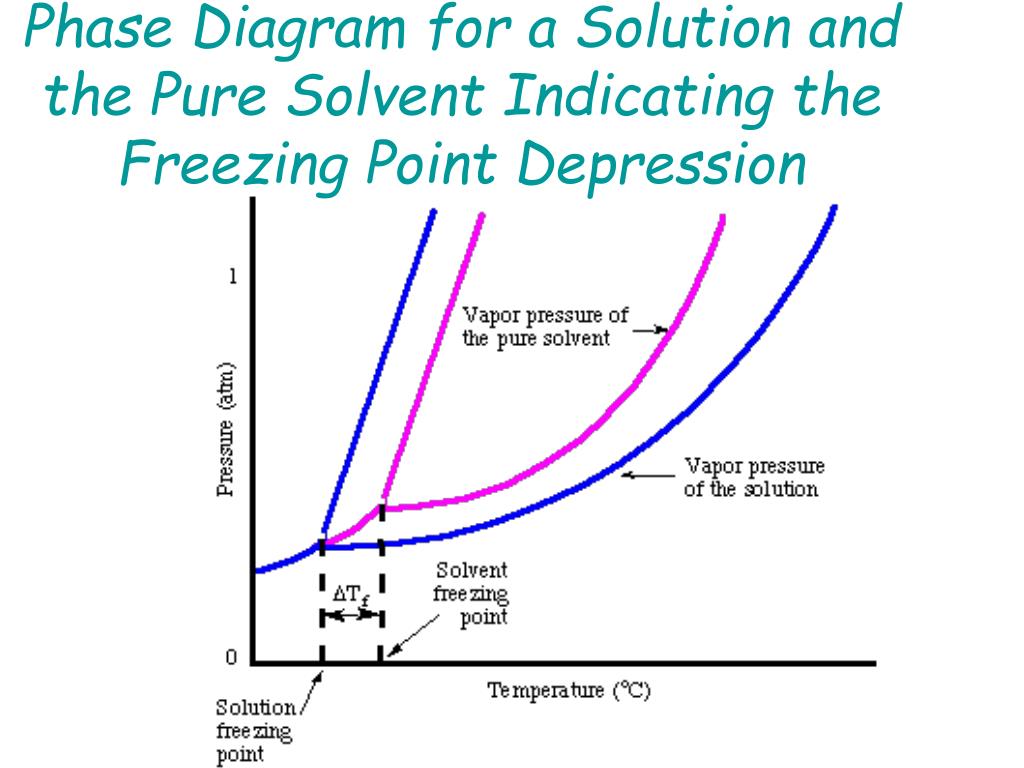
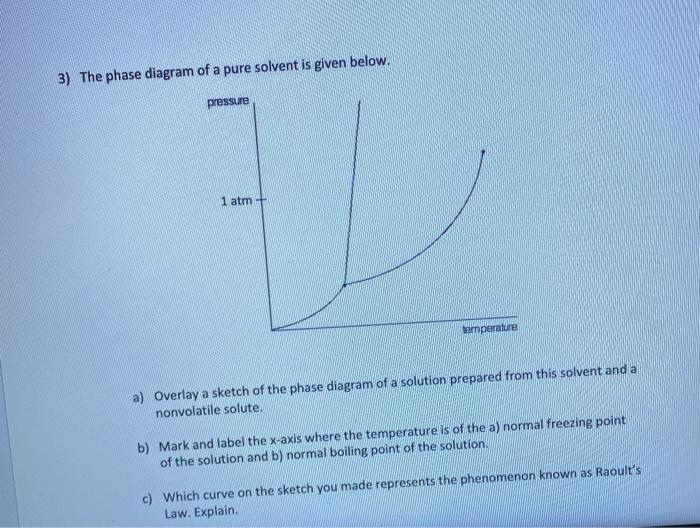
2.2: Molecular Weight Determination - Chemistry LibreTexts Mar 21, 2021 · In Equation \ref{1} the freezing point depression of a non-ionic solution is described. Where ∆T f is the change in the initial and final temperature of the pure solvent, K f is the freezing point depression constant for the pure solvent, and m (moles solute/kg solvent) is the molality of the solution. \[\Delta T _ { f } = K _ { f } m \label{1}\] Answered: Draw and completely label a… | bartleby Science Chemistry Q&A Library Draw and completely label a hypothetical phase diagram of a mixture containing menthol and camphor, which theoretically is able to form liquid eutectic at room temperature in the ratio of 8:2, 7:3, 6:4 and 5:5 : 10.4 Phase Diagrams - Chemistry A typical phase diagram for a pure substance is shown in Figure 1. Figure 1. The physical state of a substance and its phase-transition temperatures are represented graphically in a phase diagram. To illustrate the utility of these plots, consider the phase diagram for water shown in Figure 2. Figure 2. The phase diagram for solvent and solutions is shown in the figure ... Question The phase diagram for solvent and solutions is shown in the figure. What represents the normal boiling point of the solution? A A B B C C D D Hard Solution Verified by Toppr Correct option is D) The normal boiling point of the solution is that temperature at which vapour pressure of solution equals to 1 atm.
Phase Diagrams - Purdue University Phase Diagrams. The figure below shows an example of a phase diagram, which summarizes the effect of temperature and pressure on a substance in a closed container. Every point in this diagram represents a possible combination of temperature and pressure for the system. The diagram is divided into three areas, which represent the solid, liquid ...
Answered: 2. With the help of the Phase rule,… | bartleby Q: Phase diagram is a graphical representation of the physical states of a substance under different… A: While drawing phase diagram for a binary solution, we take mole fraction of the components on X-axis…
Answered: The phase diagrams for a pure solvent… | bartleby identify the normal freezing (fpsolv) and boiling (bpsolv) points for the pure solvent and the normal freezing (fpsoln) and boiling (bpgoln) points of the solution at 1 atm. assume the solute is nonvolatile and that the solid that freezes from solution is pure solvent. 1 atm liquid solid answer bank fpsolv bpsolv fpsoln bpsoln gas temperature …
CHAPTER 12, 13, & 14 study guide. Flashcards | Quizlet A solution containing 0.102 g of an unknown compound dissolved in 100. mL of water has an osmotic pressure of 28.1 mmHg at 20.°C. What is the molar mass of the compound? (R = 0.08206 L • atm/K • mol, 1 atm = 760 mmHg) ... Label the phase diagram of pure solvent and a solution....
Phase Diagrams – Chemistry - University of Hawaiʻi (b) Graphite is the most stable phase of carbon at normal conditions. On the phase diagram, label the graphite phase. (c) If graphite at normal conditions is heated to 2500 K while the pressure is increased to 10 10 Pa, it is converted into diamond. Label the diamond phase. (d) Circle each triple point on the phase diagram.
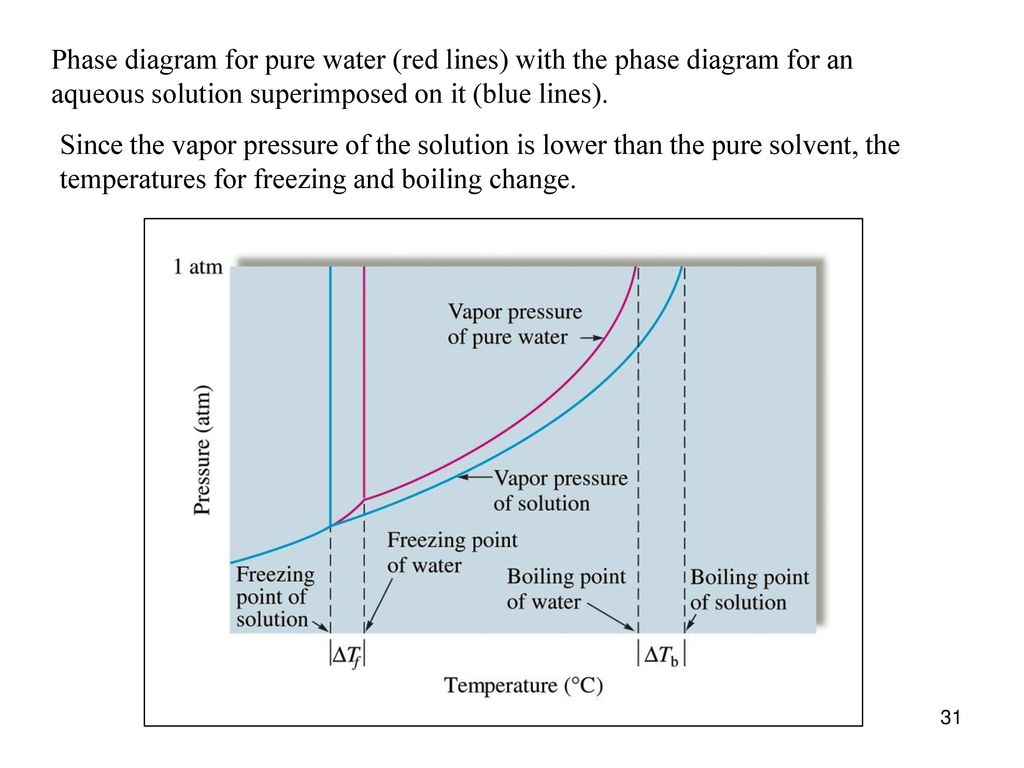
The figure shows two phase diagrams, one for a pure liquid (black line ... Similarly, the normal boiling point of the pure substance at constant pressure is indicated by C, and the raised normal boiling point of the solution with the pure liquid as the solvent (again, due to addition of nonvolatile solute to the pure liquid substance) is indicated by D, since T ↑ rightwards. ΔT b = T b − T * b = iKbm
CH103 – Chapter 8: Homeostasis and Cellular Function – Chemistry Solution = Solute + Solvent. Thus, the following equation can be used when calculating percent solutions: Example 1: As an example, a 7.0% v/v solution of ethanol in water, would contain 7 mL of ethanol in a total of 100 mL of solution. How much water is in the solution? In this problem, we know that the: Solution = Solute + Solvent
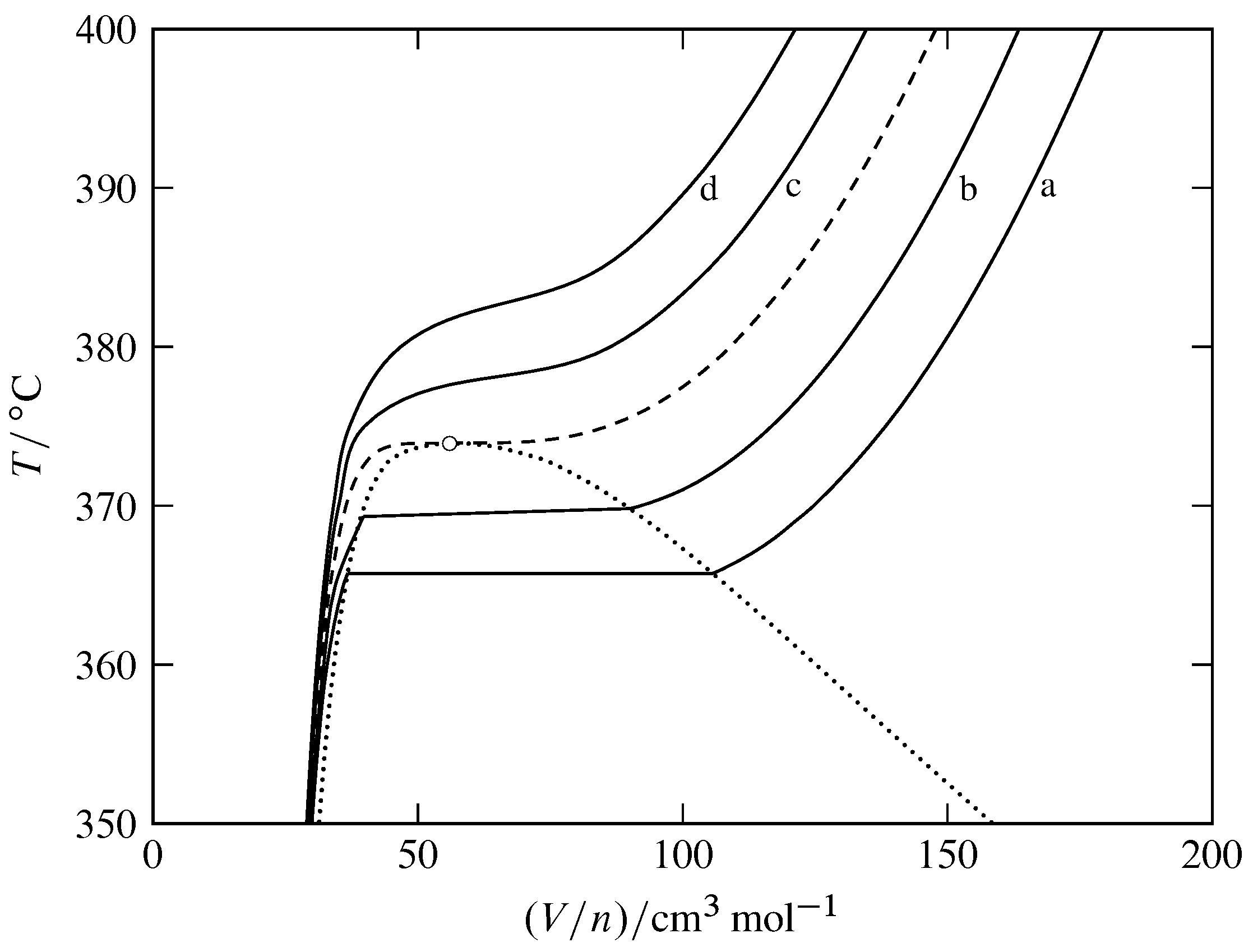
The phase diagrams for the pure solvent (solid lines) and the solution ... The phase diagrams for the pure solvent (solid lines) and the solution (non-volatile solute, dashed line) are recorded below: The quantity indicated by `L` i...
Solved PRE-LAB QUESTIONS 1. Distinguish between solute, - Chegg Distinguish between solute, solvent and solution. 2. What are the units of molarity and molality? The phase diagram for water is shown below. Label the axis and a gas, a liquid and a solid. Indicate where the triple point is located and dre Wate point. s where water is define the tiple 3. )What do the solid lines in the phase diagram
1.2 3 Separation Techniques - Save My Exams Used to separate a dissolved solid from a solution, when the solid is much more soluble in hot solvent than in cold (e.g., copper sulphate from a solution of copper (II) sulphate in water); The solution is heated, allowing the solvent to evaporate, leaving a saturated solution behind; Test if the solution is saturated by dipping a clean, dry, cold glass rod into the solution
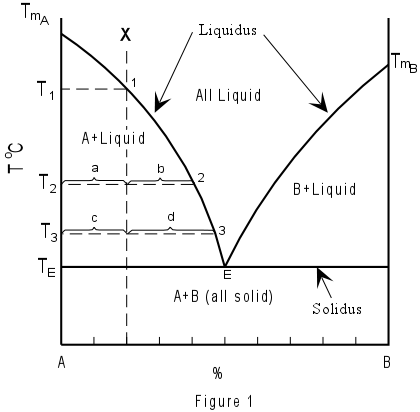
Chapter 8 Phase Diagrams - Central Michigan University The figure shows the phase diagram of a system in which the liquids become fully miscible before they boil. Distillation of a mixture at a 1 leads to vapor with composition b 1, which condenses to completely miscible solution at b 2. Phase separation only occurs when the distillate is cooled to a point in the two-phase region such as point b 3.
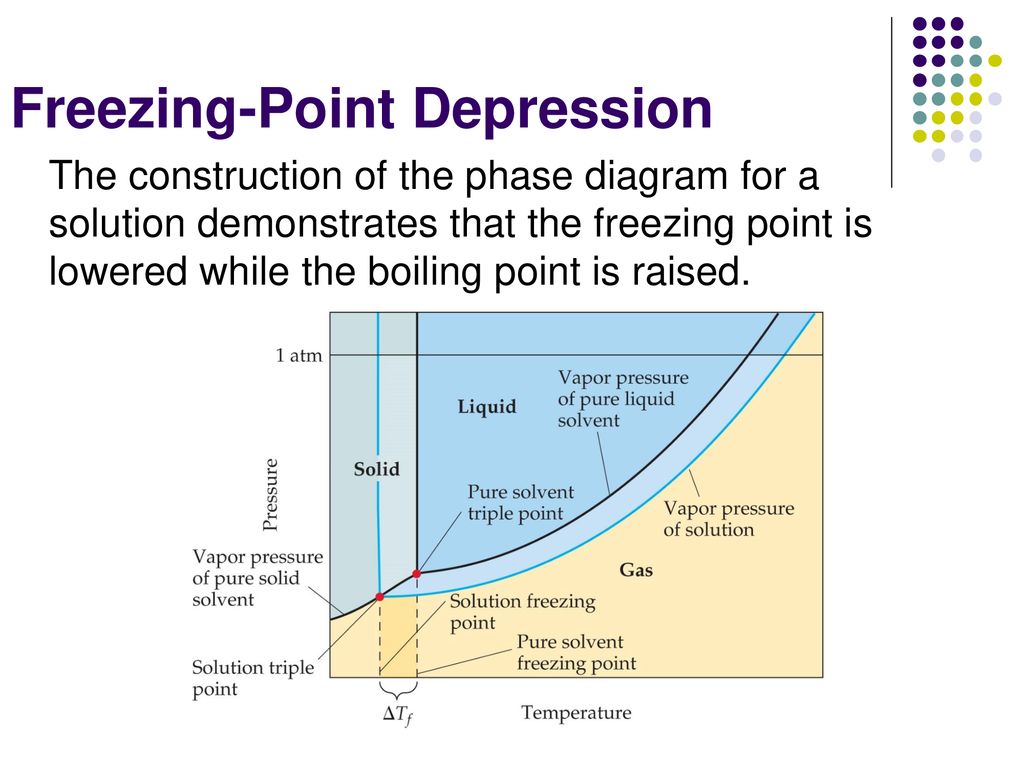
PDF phase diagram solvent solution - Just Only phase diagram solvent solution Effect of Solute on Phase Diagram of Water SOLIDLIQUID GAS Pure solvent Freezing point of solution Freezing point of water Boiling point of water Boiling point of solution 1 atm Pressure ΔTfΔTb ΔP Temperature
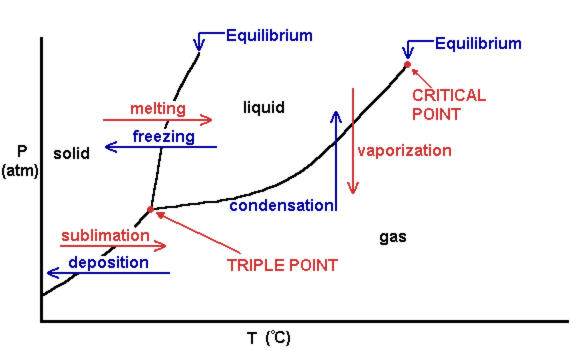
Phase diagram - Wikipedia The simplest phase diagrams are pressure-temperature diagrams of a single simple substance, such as water.The axes correspond to the pressure and temperature.The phase diagram shows, in pressure-temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas.. The curves on the phase diagram show the points where the free energy (and other ...
Ternary Phase Diagram - an overview | ScienceDirect Topics Ternary phase diagrams are used to represent all possible mixtures of three solvents [1]; they are described in Chapter 3.Here, we shall indicate how they should be used to minimize the solvent consumption. Figure 2.1 (top) shows the methanol–chloroform–water ternary phase diagram with the tie-lines in the biphasic domain. Five particular compositions are shown in the …
Phase Diagram - SlideShare Phase Diagram 1. Chapter-5 PHASE AND PHASE EQUILIBRIUM Prepared By: PALLAV RADIA Asst prof. AITS, RAJKOT. 2. Introduction: One of the most important objective of engineering metallurgy is to determine properties of material. The properties of material is a function of the microstructure which depend on the overall composition and variable such as pressure and temperature. Hence to determine ...
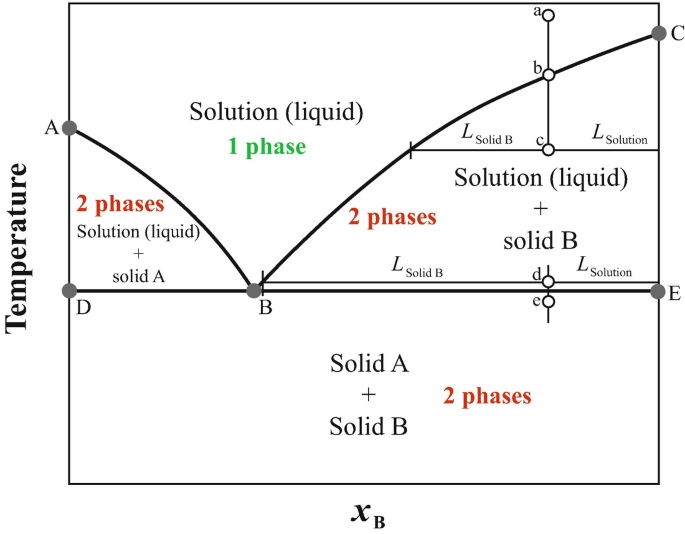
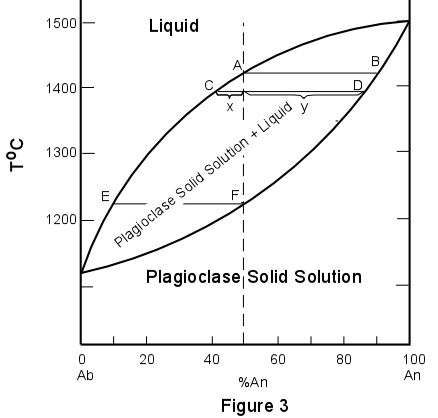
PDF Phase Diagrams, Solid Solutions, Phase Transformations Phase Diagrams: composition of phases At TA= 1320°C: Only Liquid (L) present CL= C0 ( = 35 wt% Ni) At TB= 1250°C: Both and L present At TD= 1190°C: Only Solid ( ) present C = C0( = 35 wt% Ni) C L = C liquidus ( = 32 wt% Ni) C = C solidus ( = 43 wt% Ni) 18 • Rule 3:If we know T and Co, then we know: --the amount of each phase (given in wt%).
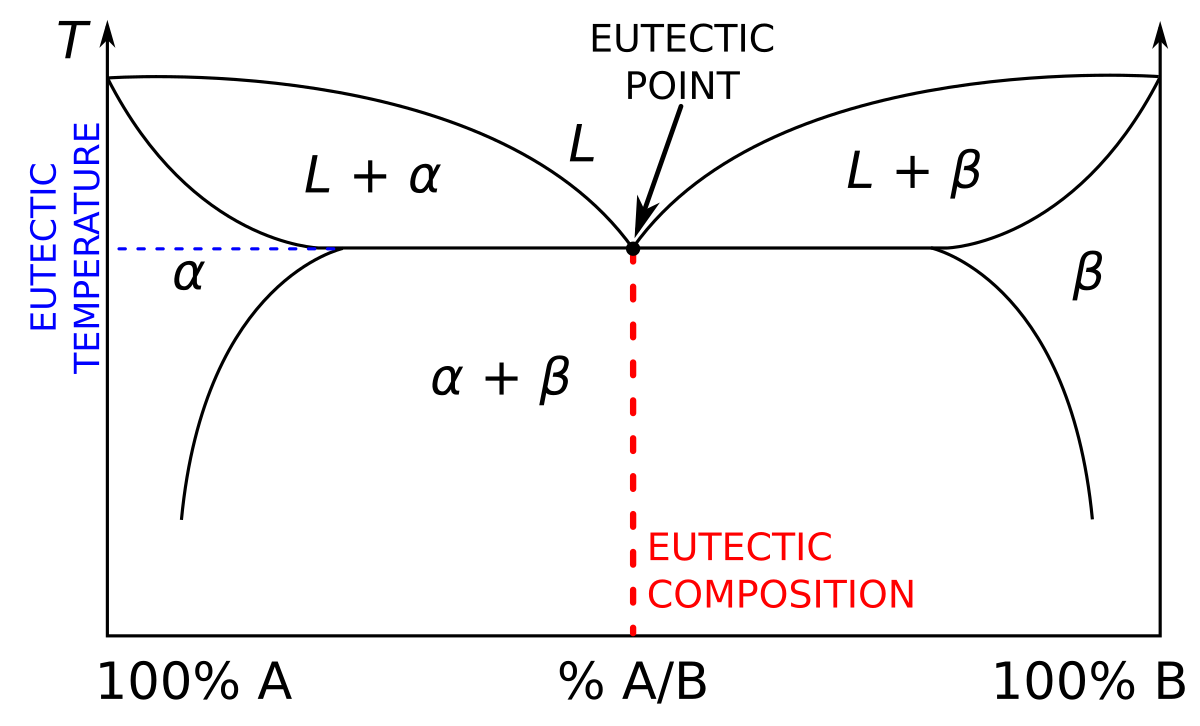
Liquid/Solid Phase Diagram - an overview | ScienceDirect Topics The (solid + liquid) phase diagram for ( x1 n-C 6 H 14 + x2 c-C 6 H 12) has a eutectic at T = 170.59 K and x2 = 0.3317. A solid phase transition occurs in c-C 6 H 12 at T = 186.12 K, resulting in a second invariant point in the phase diagram at this temperature and x2 = 0.6115, where liquid and the two solid forms of c-C 6 H 12 are in equilibrium.
Ethanol - Wikipedia Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic chemical compound.It is a simple alcohol with the chemical formula C 2 H 6 O. Its formula can be also written as CH 3 − CH 2 − OH or C 2 H 5 OH (an ethyl group linked to a hydroxyl group), and is often abbreviated as EtOH.Ethanol is a volatile, flammable, colorless liquid with a ...
Rearrangement - Michigan State University In the following diagram, the simplest hypervalent carbocation, methanonium, is drawn on the left in the gray shaded box. This ion is commonly seen in the mass spectrum of methane (gas phase), but decomposes in solution as a consequence of its extreme acidity. To its right are two larger non-classical ions, 2-norbornyl and 7-norbornenyl.
An overview on the use of additives and preparation procedure in phase … In the context of long-term energy storage, solid-to-solid PCMs are recently garnering considerable attention owing to the discovery of materials, mainly plastic crystals, with high enthalpy of transition between the solid phases .They avoid the liquid phase and offer several advantages in comparison to the solid-to-liquid PCMs, such as lack of leakages and of …
Phase Diagram | Explanation, Definition, Summary & Facts The phase diagram of a substance can be used to identify the physical and chemical properties of that substance. Here, we will study a general phase diagram by considering different values of one variable while keeping the other variable value constant. In a phase diagram temperature values are drawn on x-axis, whereas pressure values on y-axis.












Post a Comment for "39 label the phase diagram of pure solvent and a solution"